Next Generation Treatment Options for Advanced AMD
December 4, 2020
San Diego County Optometric Society Newsletter
By Nikolas J.S. London, MD FACS
President and Director of Clinical Research, Retina Consultants San Diego
Chief of Ophthalmology, Scripps Memorial Hospital La Jolla
Dear SDCOS membership,
Hey everyone! While I know 2020 has been tough, I hope all of you are doing well – back seeing patients and back to some semblance of normalcy. We are very busy at RCSD, and have several fascinating clinical trials starting. Several of the more interesting ones involve gene therapy, where you use a viral vector to enable the eye to produce a protein that it does not normally produce. In this case, medication for a retinal disease. I have written about the RegenexBio studies previously, where the treatment causes the eye to produce ranibizumab (Lucentis) to treat wet AMD. Those are starting now, and we would be happy to see anyone you think might benefit. This month, however, I wanted to write about a gene therapy study for geographic atrophy from Gyroscope Therapeutics Limited. Particularly interesting to you, possibly, is that many of you could participate if you would like.
Taking a quick step back, as you know geographic atrophy (GA) is a form of advanced AMD characterized by progressive death of the RPE and overlying retina, and accounts for about 10% of severe vision loss associated with AMD. Unfortunately we have no treatment options for affected patients, and are resigned to simply watching them lose vision. Fortunately, however, the pathogenesis of GA is fairly well-understood, and we know that a dysfunctional complement cascade plays a central role. This is the target of most investigational therapies – inhibiting an overactive or dysregulated complement cascade. While several treatment options have tried and failed, gene therapy holds significant promise as it may enable permanent and constant production of a protein.
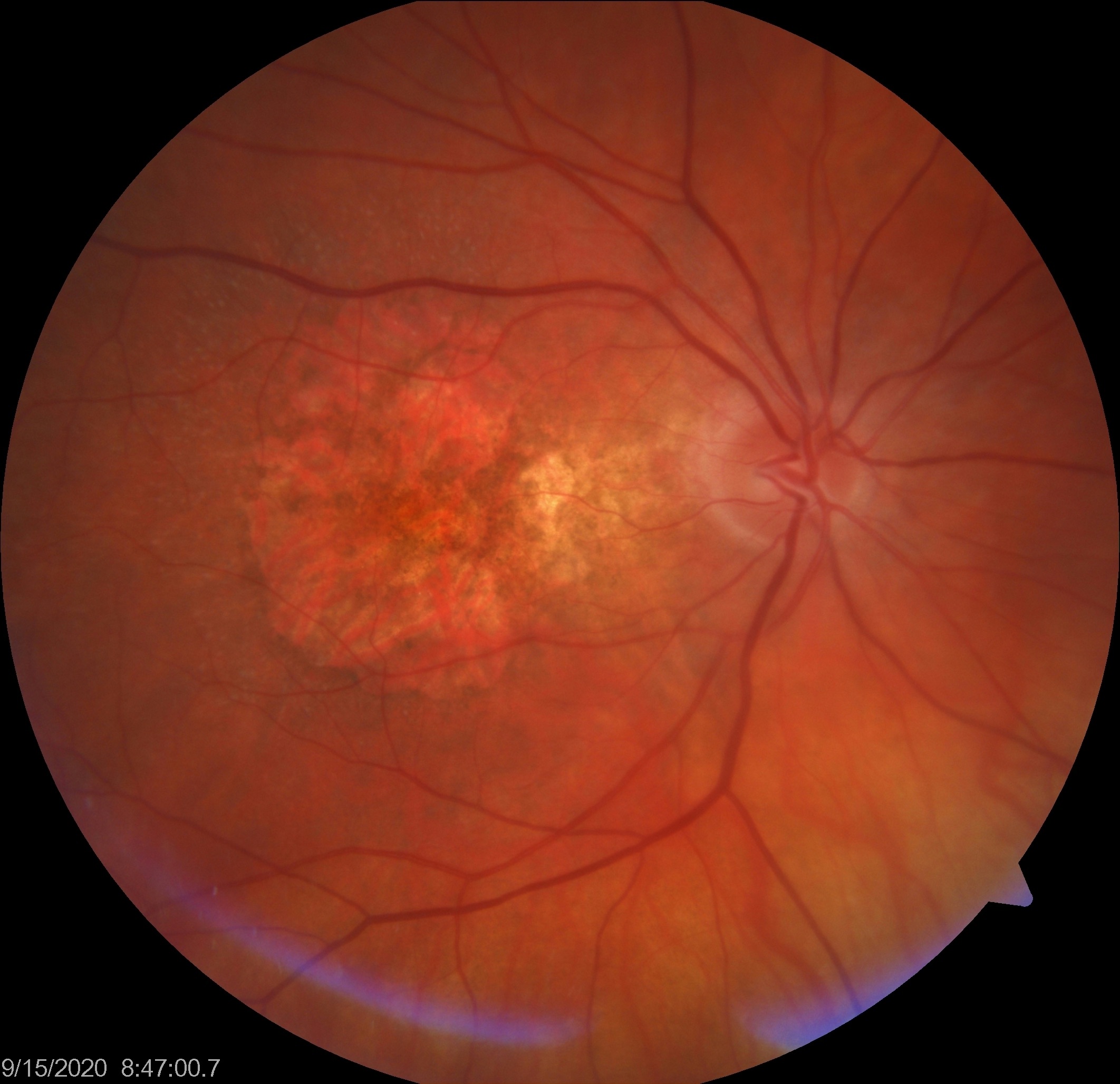
Gyroscope Therapeutics Limited is a biotechnology company out of London, England that focuses on gene therapies. They have a promising treatment option for GA secondary to AMD that uses a benign viral vector (AAV2) to elicit sustained production of complement factor I (CFI), which is an important down regulator of the alternative complement system. Some AMD patients are genetically-insufficient in CFI, and have a very high risk of advanced AMD, including GA formation. These patients are the initial target of this study – a small group of patients with a relatively rare genetic variant (CFI insufficiency). Enrolled patients would potentially receive the gene therapy as a subretinal injection, and would hopefully have a much lower risk of advanced AMD.
This is where you may come in, if you are interested. A significant initial part of the study involves screening patients with GA for this rare genetic variant. This involves a simple cheek swab or blood draw. We will be doing this for our GA patients, and if you are interested and see patients with GA, the Sponsor would like work with you as well, and would compensate you several hundred dollars per patient tested. I would be happy to put anyone interested in touch with them.
Well, I think that is about it for this month. Thanks so much for reading. Please don’t hesitate to contact me with any questions. And let me know about the doggy playdate!
Best wishes, and until next time,


